Filters
Standard
Type
Topic
clear search
Filters
clear filters
Standard
Type
Topic

Articles
ISO 13485
Design and development validation and verification according to ISO 13485
by Chany Runnels

Articles
ISO 13485
Production and service provision process in ISO 13485
by Strahinja Stojanovic

Articles
ISO 13485
How to manage the medical device sterilization process according to ISO 13485:2016
by Waqas Imam
Articles
ISO 13485
ISO 13485:2016 nonconforming product – How to approach the post-delivery actions
by Waqas Imam

Articles
ISO 13485
How to comply with the latest changes in ISO 13485 clause 7.2.3 Communication
by Verlene Law

Articles
ISO 13485
How to determine regulatory requirements according to ISO 13485:2016
by Waqas Imam

Articles
ISO 13485
What are the consequences of noncompliance with ISO 13485 for manufacturers of medical devices?
by Waqas Imam

Articles
ISO 13485
How to use ISO 13485 to fulfill FDA regulatory classes for medical devices
by Waqas Imam

Articles
ISO 13485
How to use ISO 13485 to comply with In Vitro Diagnostic medical devices (IVD) requirements in UK
by Waqas Imam

Articles
ISO 13485
Can determining the context of the organization be beneficial for ISO 13485 implementation?
by Waqas Imam

Articles
ISO 13485
Calibration requirements in ISO 13485
by Anita Joshi
Articles
ISO 13485
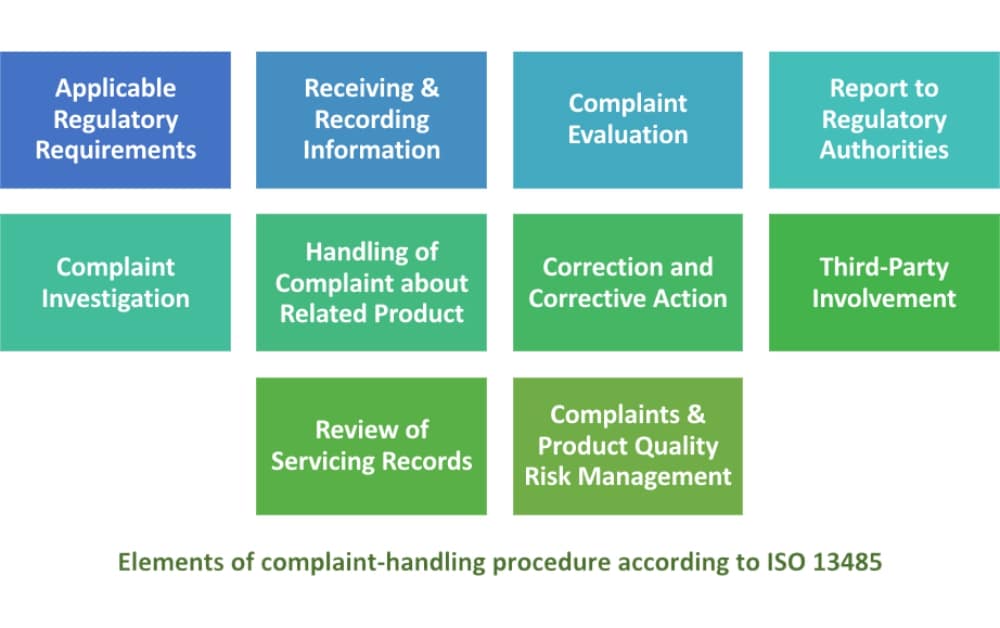
How to comply with ISO 13485:2016 requirements for handling complaints
by Waqas Imam
Articles
ISO 13485
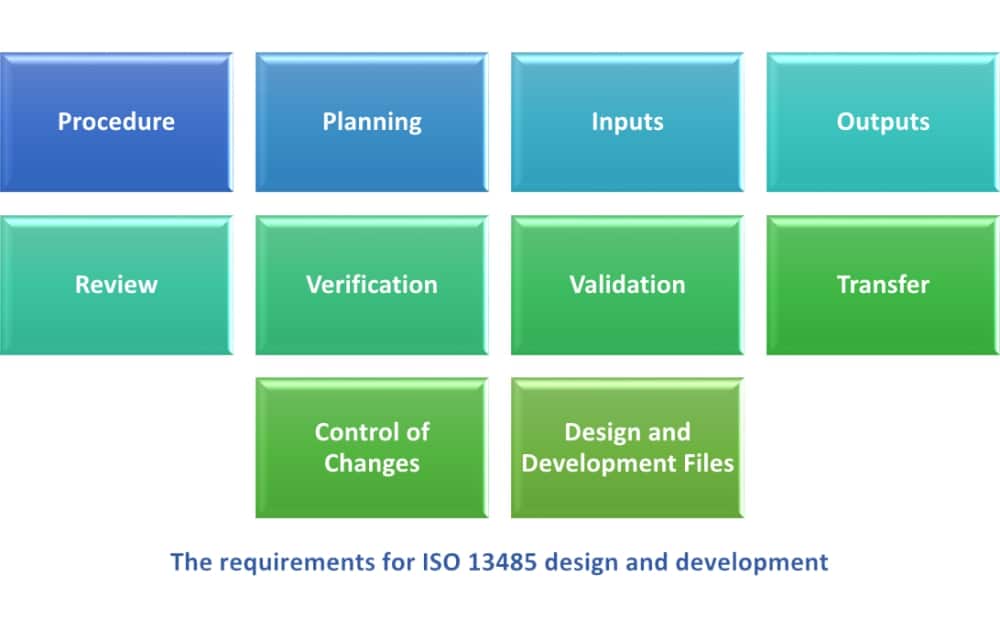
How to manage design and development of medical devices according to ISO 13485:2016
by Waqas Imam

Articles
ISO 13485
Managing cleanliness of a product and contamination control according to ISO 13485:2016
by Waqas Imam

Articles
ISO 13485
How to manage recalls and advisory notices for medical devices according to ISO 13485
by Waqas Imam

Articles
ISO 13485
Using ISO 13485 to manage process validation in the medical device manufacturing industry
by Waqas Imam
Articles
ISO 13485
How to define roles and responsibilities within an ISO 13485-based QMS
by Strahinja Stojanovic

Articles
ISO 13485
How to perform management review according to ISO 13485
by Strahinja Stojanovic
Sort by:
We couldn't find anything
Try adjusting filters or let us help you quickly find what you need by answering a few questions.