The European Commission published a step-by-step guide in 2018 to help medical device manufacturers prepare for regulatory change. In the guide you’ll see below, we explain how to comply with the EU Medical Device Regulation (MDR) to demystify the process and help manufacturers prepare the necessary documentation for MDR compliance.
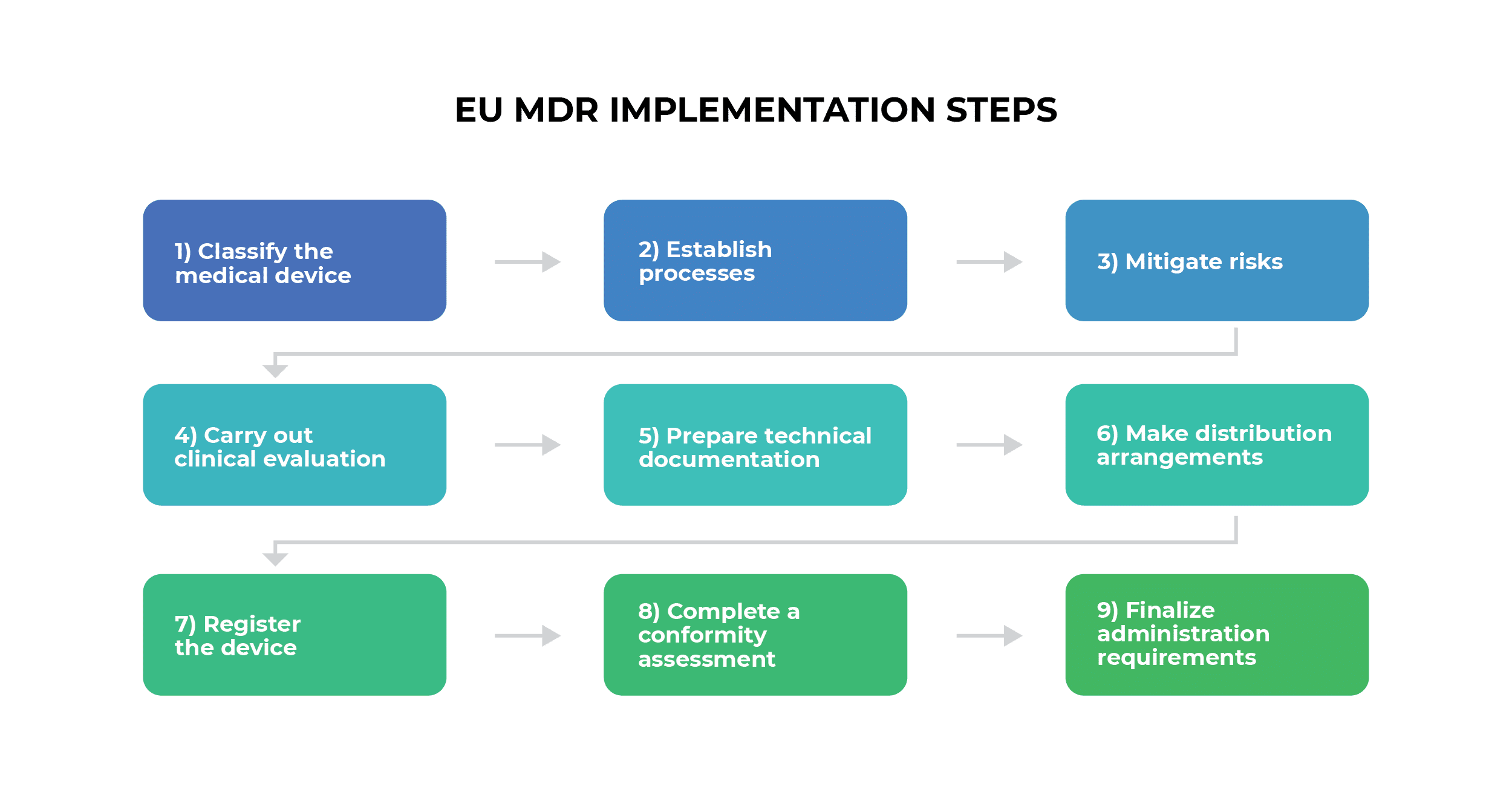
- Classify the medical device
- Establish processes
- Mitigate risks
- Carry out clinical evaluation
- Prepare technical documentation
- Make distribution arrangements
- Register the device
- Complete a conformity assessment
- Finalize administration requirements
Step 1: Classify the medical device
First, a manufacturer needs to determine if their product aligns with the definition of a medical device as stated in MDR Article 2. The classification then needs to be determined in accordance with Article 51 and the rules contained in Annex VIII.
When classifying devices, a manufacturer needs to:
- Review the definitions and specify which are relevant to their medical device
- Decide which implementing rules are necessary; for instance, if the device is to be used for different body parts or conditions
- Work through 22 classification rules to classify their device
If a manufacturer has been unable to classify their device, they can write to their Competent Authority and ask for their opinion. But ultimately, the manufacturer has to make the decision.
Learn more about how manufacturers classify medical devices: Medical device classification according to the EU MDR.
Step 2: Establish processes
The next step manufacturers should carry out is to ensure that their processes are compliant with the MDR framework. These processes are covered in MDR Article 10, and some of them include:
- Design and manufacturing
- Risk management
- Clinical evaluation
- Technical documentation
- Quality Management System (QMS)
During this stage, a manufacturer should consider whether they have the internal capabilities to carry out these processes, or they need to recruit or subcontract to specialists in the field.
Step 3: Mitigate risks
Once the processes have been established, device manufacturers must ensure that they adhere to the safety requirements listed in MDR Annex I. This offers a high-level summary of European general safety and performance requirements.
This annex is divided into three chapters:
- Chapter I – General requirements
- Chapter II – Requirements regarding design and manufacture
- Chapter III – Information supplied with the device
Manufacturers should start with planning the various risk management activities listed in Chapter I, such as “informing users of any risks” and “eliminating risks related to use error.”
After this, it’s important to read through Chapter II, make a list of requirements for the medical device, and provide evidence that these have been satisfied. Then, in Chapter III, the same process must be carried out in relation to the information supplied with the device, such as instructions and labeling.
In terms of risk management, the final stage is to prepare a post-market surveillance plan (as referenced in Article 84 and Section 1.1 of Annex III.
Read this related article for expert risk management advice: How to use ISO 14971 to manage risks for medical devices.
Step 4: Carry out clinical evaluation
After the risk management activities have been taken care of, a clinical evaluation must be carried out based on a new evaluation or an equivalent existing certified medical device (as referenced in MDR Article 61 and Part A of Annex XIV), making use of either:
- Published literature
- Clinical investigations
To figure out which route to take, a manufacturer needs to carry out a clinical evaluation plan, which seeks to identify the existing data. If more information is needed, it can be gathered through a clinical investigation in accordance with Articles 62-81 and Annex XV.
Once the clinical data have been evaluated and summarized, the manufacturer can prepare their post-market clinical follow-up (PMCF) plan, as outlined in Annex XIV, Part B.
Read more about clinical evaluation documentation: How to write clinical evaluation reports under the EU MDR.
Step 5: Prepare technical documentation
By this point, a medical device manufacturer will have compiled lots of information, which needs to be organized to show that the medical device:
- Conforms to the EU’s technical requirements
- Achieves its intended medical purpose
- Is safe to use
Manufacturers should compile a checklist of the technical documentation that’s included in MDR Annex II and Annex III.
A unique device identifier (UDI) is needed to complete the technical documentation, which is essentially a barcode that allows devices to be tracked in the EU.
Learn more about the technical documentation required for MDR compliance: What are the EU MDR technical documentation structure and requirements?
Step 6: Make distribution arrangements
A manufacturer needs to consider how their medical device will be distributed across EU member states. They also need to ensure that their distributors have the information they need to comply with the EU MDR.
Anyone who imports a medical device into the EU is obligated to register with authorities and the European Database of Medical Devices (EUDAMED), and they must also display their name and address on the product in accordance with MDR Article 13.
Manufacturers based outside of the EU need to appoint an authorized representative in the EU to act on their behalf.
Step 7: Register the device
After a manufacturer has made the necessary distribution arrangements, they can proceed with registering the device, which involves two stages:
- Submitting the economic operator information (MDR Annex VI, Part A, Section 1).
- Submitting the device information (Annex VI, Part A, Section 2) and UDI data.
After submitting the economic operator information, the manufacturer (or importer) is issued a single registration number (SRN) that is visible in the EUDAMED database. With the SRN, the manufacturer can then complete the EU declaration of conformity (Annex IV) and submit the application to the Notified Body.
The manufacturer must register their medical device as outlined in Article 29. This can only be done after the device receives a CE marking.
Step 8: Complete a conformity assessment
Manufacturers can decide which Notified Body will carry out the conformity assessment. If a device doesn’t require a Notified Body to carry out a conformity assessment (such as class I devices), manufacturers can skip Step 8.
There are a few different routes that manufacturers can take. MDR Annex IX contains the conformity assessment route for manufacturers who develop, manufacture, and support the device in service. If successful, the Notified Body issues:
- An EU quality management system certificate
- An EU technical documentation assessment certificate
Manufacturers who do not fulfill the QMS requirements outlined in Annex IX can proceed to Annex X. The Notified Body will issue an EU type examination certificate after they have been provided with technical documentation, clinical evidence, and samples of the device for testing.
Learn more about what to do when medical devices are deemed nonconforming: ISO 13485:2016 nonconforming product – How to approach the post-delivery actions.
Step 9: Finalize administration requirements
After receiving their certificates from the Notified Body, non-EU manufacturers must provide their authorized representative with technical documents, certificates, and registration confirmation. The representative will then need to register in accordance with MDR Article 11.
As per Article 13, all manufacturers are obligated to provide importers and distributors with additional information, such as the EU declaration of conformity and device labeling information.
Once all the key stakeholders have the required documentation, the manufacturer can market, distribute, and supply the product within the EU.
Read about the challenges and requirements of labeling medical devices: How to comply with the MDR requirements for medical device labels.
EU MDR: A continuous process
Once you have received your CE certificate, there is still more work to do. Now you can freely sell your product on the EU market, but you must also be committed to various types of monitoring and keeping technical documentation up to date. A well-established MDR system will make your job easier.
To set your documents and policies compliant with the EU MDR in the fastest and most efficient way, check out this EU MDR & ISO 13485 Integrated Documentation Toolkit.

 Kristina Zvonar Brkic
Kristina Zvonar Brkic