A key part of implementing a Quality Management System (QMS) based on IATF 16949 is developing the documentation and a system for record control. You need to make sure your organization creates the most suitable documentation, as it will define the way your QMS is maintained and improved – as well as specifying how your documents and records must be created, published, withdrawn, and used. It’s a question of: Do you want your documentation to be just a formality, an inconvenience that makes your QMS unusable? Or, would you like your documentation to make it easy to maintain your QMS, allowing your company to enjoy the maximum benefit of the IATF 16949 implementation?
Which documents are most important?
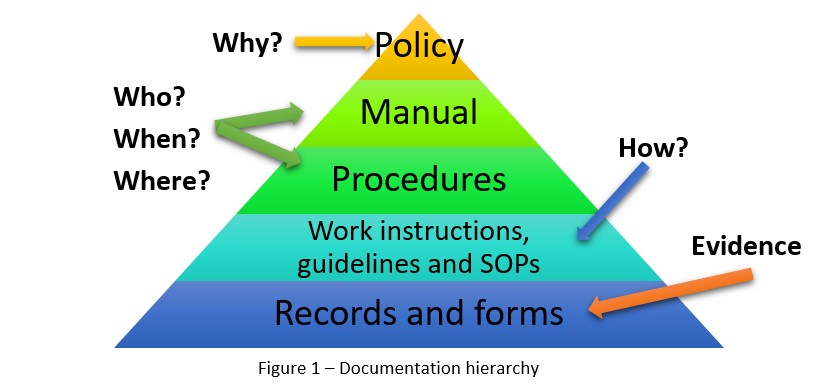
Before you sit down to create your QMS documentation, you should first have a good understanding of each document, its purpose, and its place in the documentation hierarchy. Establishing a Quality Management System requires lots of documentation, including a quality policy, quality manual, procedures, work instructions, guidelines/Standard Operating Procedures (SOPs), records, and forms. Each document and record type has its own role in the QMS, and its place in the documentation hierarchy. Figure 1 provides an illustration:
Oftentimes, when people are just getting started on the creation of their documentation, they become confused about what order the documents should go in, and which is more important than the others. The best way to figure this out is to look at who writes a document, who they write it for, and for what purpose. Then, you can start filling in the hierarchy: if someone in top management wrote the document, then it goes at the top of the pyramid, while if it was written by employees, it should go at the bottom.
Structuring your QMS documentation
IATF 16949 requires certain documents as a part of the Quality Management System (see this List of mandatory documents required by IATF 16949 to learn more).There are multiple reasons for this mandatory documentation:
- to give a clear overview of the company operations
- to provide a better understanding of the QMS
- to promote consistency in processes
- to show proof of achieving goals and objectives
During the development of your QMS documents, it’s important that you focus on what you company actually needs to document: What documents can help increase efficiency? What documents are applicable to your company, and the industry it operates in? The last thing you want is a bunch of extra documents that no one uses, because they just aren’t relevant.
So, to this end, make sure to identify the role and purpose of each document before creating and implementing it. Here’s how to structure your IAF 16949 Quality Management System documentation:
1) Quality manual. The quality manual needs to be tailored to your company – its structure and content should be based on your company size, operational complexity, and employee competence. Smaller companies will be able to fit their whole QMS into one manual, but larger companies with several locations will likely have a number of separate quality manuals. In general, the quality manual will contain these basic elements:
- document title
- table of contents
- QMS scope and exclusions
- information on version and approval
- description of the Quality Management System
- the company’s business process model
- definitions of employee responsibilities
- references to relevant documents and appendices
2) Quality policy. In general terms, “policy” refers to a statement of assertion made by an organization. A quality policy should make a statement about the company’s commitment to quality and continual improvement of its processes. This policy should be brief, concise, and to the point, because it is often used for promotional purposes, visible on the company premises and posted on its website.
The purpose of the quality policy is to define the company’s framework for achieving quality objectives. In turn, the company’s quality goals are defined through quantifying these quality objectives.
3) Procedures. QMS procedures should contain the document title, its purpose and scope, responsibilities and authorities, descriptions of all activities, and references to any relevant SOPs, work instructions, and records. How you document this information is up to you: procedures can be in narrative form, illustrated through diagrams and flow charts, structured using tables – or, you can use a combination of formats. You may also include appendices, if necessary.
4) Work instructions. You may include work instructions in a procedure, or simply refer to them in the procedure. Work instructions usually include the same elements as the procedures, and are similar in structure. The difference is that the work instructions are more detailed, covering activities that must be done, the steps to take and in what order, what tools and methods should be used, and accuracy requirements.
5) Records and forms. To sum it up, you will need to provide proof that your activities and processes are done in the manner that is specified in the procedures and the work instructions. This is where records and forms come into play. The majority of these will be completed by employees, though a few will be the responsibility of top management (for example, Management Review Minutes). Focus on creating short, easy-to-complete records and forms to keep them practical (and keep personnel using them); try checkboxes rather than open-ended questions and empty rows.
Your QMS depends on good documentation
The QMS documentation must be structured according to the needs of your company if you want it to be practical and effective. Taking the time to create your documents the right way will make your processes easier, better, and more efficient. Rushing to simply get the documentation finished will only cause trouble in the long run.
To find out what documentation is required by IATF 16949 and what is commonly used, see this white paper on Mandatory Documentation Required by IATF 16949:2016.

 Strahinja Stojanovic
Strahinja Stojanovic